Introduction
The science component of the TEAS exam is intended to assess the candidate’s scientific skills and knowledge, and chemistry is one of the subsections assessed. The chemistry section assesses the candidate’s understanding of fundamental chemistry concepts such as chemical processes, matter, and stoichiometry. To pass this section, your TEAS test chemistry preparation must be thorough. A robust performance in this section can enhance your overall TEAS test scores for higher chances of qualifying.
Study Strategies for TEAS Test Chemistry Preparation
Your TEAS test chemistry preparation should have a robust study strategy to help manage your time correctly. Below are some brilliant TEAS chemistry section tips to implement when preparing:
- Start with the basics by looking at the fundamental chemistry concepts before exploring the complex problems. By doing the easy ones first, you will tackle as many questions as possible.
- Use flashcards – for the crucial concepts, equations, and key terms, use flashcards to help with memorization. They help a lot in the retention of information.
- Practice problem-solving regularly – work on different problems regularly to enhance your problem-solving abilities. Your preparation will make you more comfortable and confident during the exam.
- Use mnemonics and visual aids – use mnemonic devices, charts, and diagrams to visualize the important concepts in chemistry. They will help remember information, especially the structures of molecules.
- Form study groups—You could also form groups with peers to discuss challenging topics or motivate and push each other to improve.
- Utilize online resources—Explore the online resources and platforms specifically created to offer TEAS test assistance. These resources may include practice questions, quizzes, and explanations on various chemistry topics. A good example is the ATI TEAS website, which offers multiple study guides and materials.
- Seek help – you can consider seeking help from peers and tutors on challenging topics. It is another effective strategy since these experts will explain every concept or formula in black and white as you ask them questions.
- Use concept maps. Concept maps help connect the dots in crucial chemistry concepts. These visual tools help represent the relationship between theories, ideas, elements, and reactions.
- Practice, practice, practice—one of the surest ways to retain information is to go through it repeatedly. Take as many practice tests in the chemistry section as possible to let the concepts and formulas sink in.
Common Challenges Faced By Test-Takers
The common challenges you will encounter are understanding complex chemical reactions, identifying ionic and covalent bonds, balancing chemical equations, and describing chemical reactions.
Some students may also struggle to apply knowledge of acids, bases, essential molecules, and pH balance in context. To overcome these challenges, endeavor to explore the above chemistry topics and use reliable resources to practice. You could also ask for help from peers or tutors whenever you are stuck.
Resources To Use During TEAS Test Chemistry Preparation
Even though the TEAS test is quite challenging at times, there are numerous resources students can take advantage of. This TEAS chemistry review guide section covers essential resources you can use to pass your exams.
- Textbooks and study guides—Nursing aspirants can use several reliable chemistry textbooks to prepare for their TEAS test. A good example is the ATI TEAS 7 secret study guidebook or the ATI TEAS 7th Edition study guide.
- Online courses and tutorials – you could also look for reputable online courses and tutorials that offer comprehensive chemistry courses. One such course is found in Coursera. It is a reliable website that offers diverse courses to aspiring student nurses.
- Practice tests and question banks are impeccable TEAS chemistry study resources since they equip students with the requisite exam skills. Look for reliable sites like Union Test Prep, Nursing Prep Exams, or Gentoo Labs.
Understand The Chemistry Section
The TEAS exam’s science section contains 44 scored and six unscored questions, while the chemistry segment contains eight scored questions. Students have 60 minutes to complete 50 questions on various science topics and concepts, including chemical reactions and compounds. To excel in this section, you need to understand the topics covered and the relevance of chemistry in the healthcare field. You also need to know the common challenges you will likely face and how to deal with them.
Topics Covered In The Chemistry Section
Understanding the TEAS test chemistry concepts in as many topics as possible sets you up to succeed in the exam. Here is an overview of the examinable issues you can expect in the chemistry section:
1. Phase changes
This topic discusses different matter-phase changes, such as freezing, condensation, vaporization, sublimation, and melting. Phase changes typically happen when pressure or temperature is altered, ultimately affecting how the molecules interact. These changes entail energy transformations, with some processes being exothermic (freezing) and others being endothermic (sublimation).
2. Chemical bonds
The topic of chemical bonds discusses the fundamental principles of chemical bonding and examines the types of bonds between atoms and molecular shapes. Ensure you explore the different subsections, including covalent bonds, the formation of ionic bonds, atomic structure, bonding, and intermolecular forces.
3. Periodic Table of Elements
An effective TEAS chemistry preparation strategy has to explore the periodic table of elements. It consists of elements with different chemical properties. As you prepare for the TEAS test, review the element classification, periodic law, element properties, periodic trends, and element classification. These subsections on the topic will give you a comprehensive understanding of the properties, organization, and trends of different elements, as shown in the periodic table.
4. Acids and Bases
When studying acids and bases, start with their properties, such as their reactivity to indicators, conductivity, and pH. Explore acid-base reactions, pOH and pH, titrations, acid and base strengths, and buffers. Also, understand that acids typically donate protons, while bases accept protons. Bases are bitter-tasting and accept protons or donate a pair of valence electrons to create a bond. Acids taste sour and turn blue litmus paper red. They also neutralize bases to create salts.
5. Catalysts and Enzymes
Catalysts increase the reaction rate of a substance without being consumed, while enzymes are particular biological catalysts. The topic of catalysts and enzymes covers enzyme kinetics, regulation, inhibition, and applications. Ensure you understand how catalysts are crucial for maintaining life processes by accelerating reactions and how enzymes reduce the activation energy required for reactions, thus speeding up essential processes in living things.
Try some example questions from the TEAS practice test to familiarize yourself and learn how to answer them correctly.
Chemistry is critical in healthcare since it contributes to developing medical equipment, pharmaceuticals, and diagnostic processes. Chemists are responsible for researching, developing, and testing medications to ensure their safety in inpatient use.
Essential Chemistry Concepts for TEAS Test Preparation
This section covers the TEAS test chemistry concepts you must examine when revising for the exam. These concepts are examinable, and you will most likely encounter a question or two about them.
1. Atomic Structure and The Periodic Table
An atomic structure contains a nucleus where the neutrons and protons are found. The electrons that revolve around the nucleus can also be found here. Neutrons have no charge, while protons are positively charged subatomic particles. Electrons, on the other hand, are negatively charged and are much smaller than neutrons or protons.
As you work on your TEAS test chemistry preparation, do not forget to familiarize yourself with the periodic table. From studying this table, you will better understand how elements behave and relate to one another. The periodic table organizes elements based on their properties, allowing students and scientists to predict the characteristics of these elements and how they react.
2. Chemical Bonding and Molecular Structure
You need to understand the types of chemical bonds and their entailments fully. Three types of chemical bonds are metallic, covalent, and ionic.
Ionic bonding involves the transfer of an electron from one atom to another, which results in the attraction of ions with opposite charges. Covalent bonding involves two atoms that share electrons, forming a new orbit around both atoms’ nuclei and creating a molecule. Metallic bonding is when metal atoms bond to form a crystal lattice structure that conducts heat and electricity.
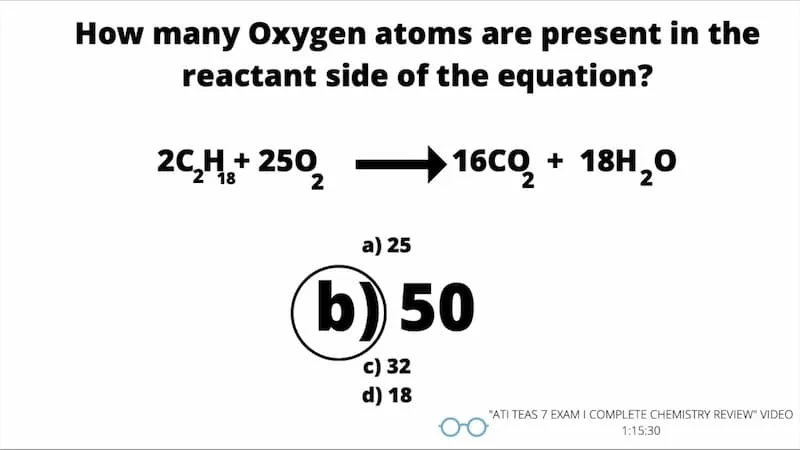
3. Chemical Reactions and Stoichiometry
Balancing chemical equations entails balancing the number of atoms on both sides of the equation. Balancing equations is vital since it obeys the law of conservation of mass, which holds that matter cannot be destroyed or created in a chemical reaction.
You could use conventional or algebraic balancing methods to balance a chemical equation. In the traditional method, you must obtain the unbalanced equation and then assign stoichiometric coefficients to the products and reactants to balance the total number of atoms.
Molar mass and mole ratio are among the key concepts for TEAS chemistry that students should look at when preparing for the exam. To calculate molar mass, you only add the molar masses of component atoms in a specific substance. Molar mass means the mass in grams of 1 mole. With this information, you are one step closer to passing the science section for the TEAS test.
4. Acids and Bases
These components showcase different properties that exhibit their differences. Acids have below-7 pH values, are corrosive, and have a sour taste, while bases have above-7 pH values, are slippery, and have a bitter taste. Acids usually turn blue litmus paper red, while bases turn red litmus paper blue.
It is also crucial to understand the pH scale. It measures the basicity or acidity of a solution. The scale ranges from 0 to 14, with a pH OF 7 being neutral, above 7 being acidic, and below 7 being basic. Polish up on this topic to have an easy time during your TEAS test.
Conclusion
The TEAS test is essential to your nursing career as it determines whether you will join your dream institution to become a decorated nurse. That is why it is crucial to focus on the test and prepare well. The chemistry section, in particular, is somehow challenging for most students.
Consider understanding the key concepts in chemistry and have robust strategies to handle the challenging questions to have an easy time. Remember to implement what you learned in this TEAS chemistry review to succeed in your exam.
